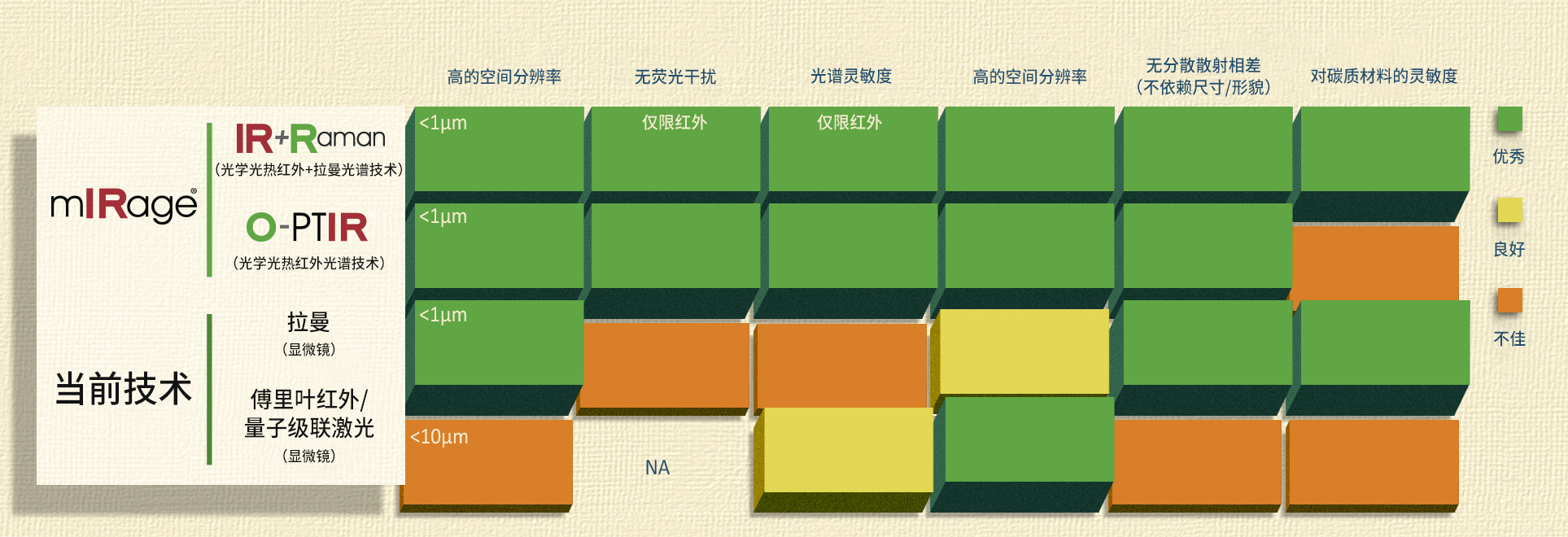
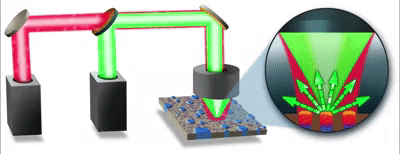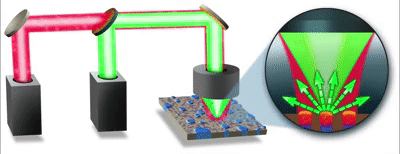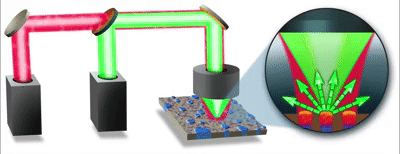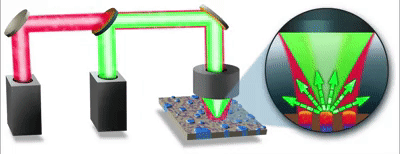
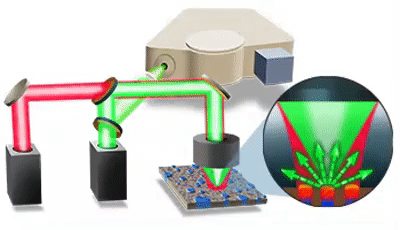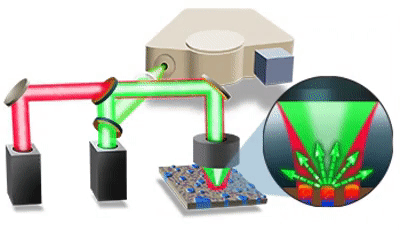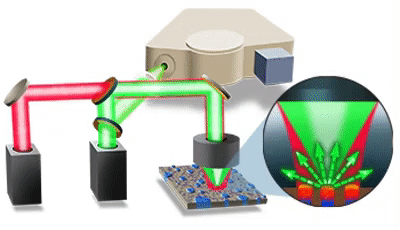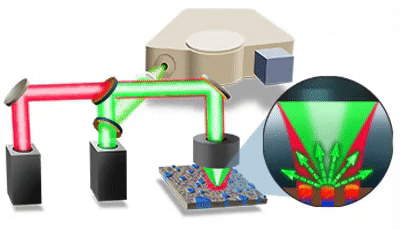
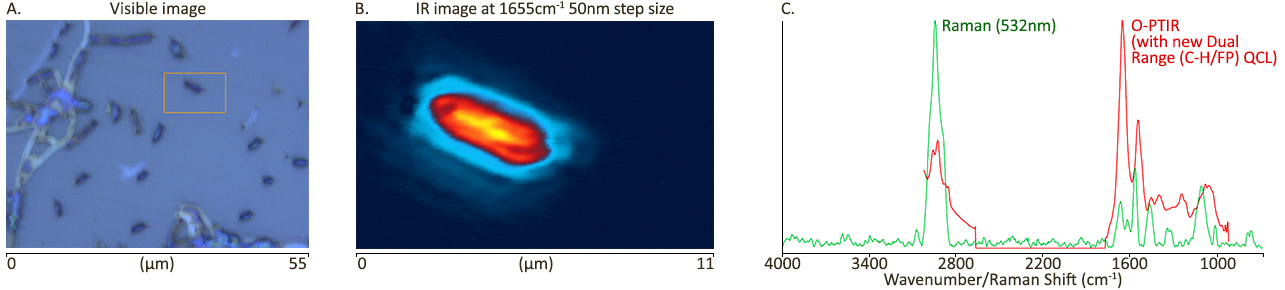
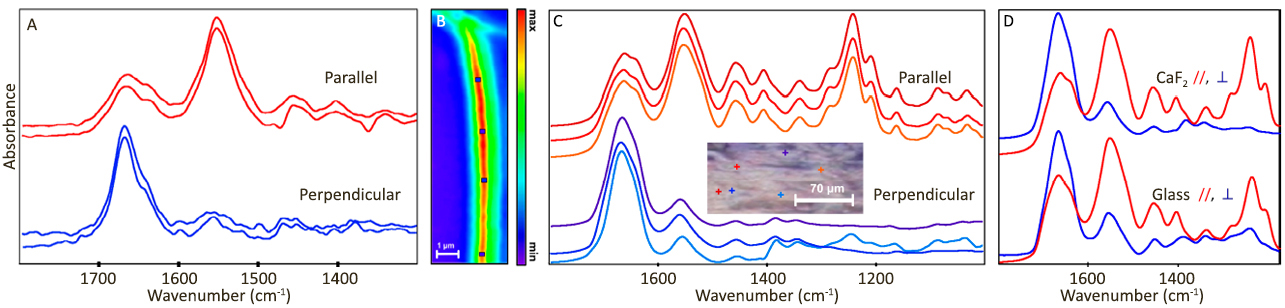
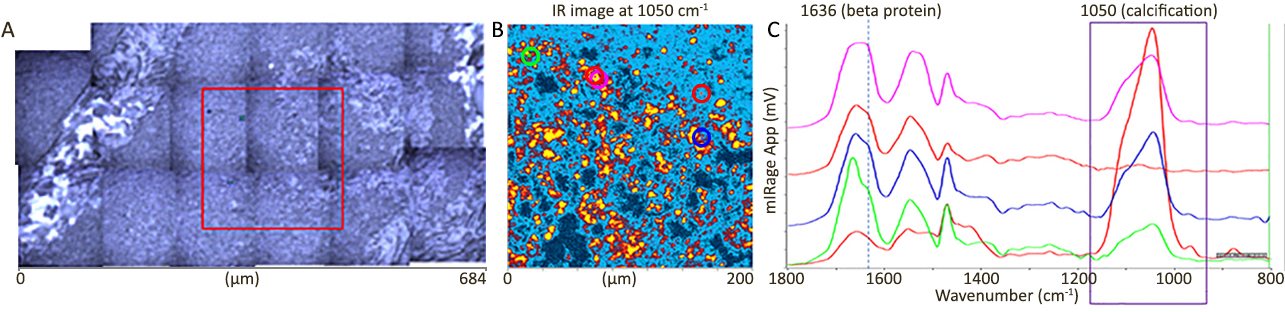
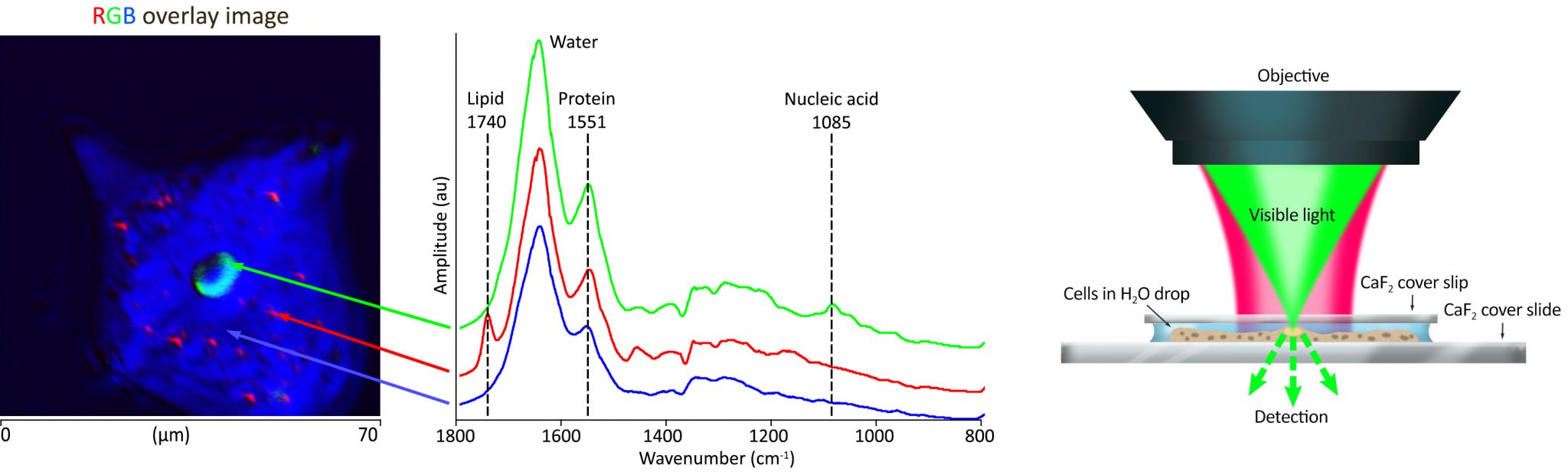
| 发表文章 | 应用领域 |
| Optical photothermal infrared spectroscopy for nanochemical analysis of pharmaceutical dry powder aerosols. Khanal, D. et al.International Journal of Pharmaceutics, 2023 | Pharmaceuticals |
| Fluorescently Guided Optical Photothermal Infrared Microspectroscopy for Protein-Specific Bioimaging at Subcellular Level. Prater, C et al.Journal of Medicinal Chemistry, 2023 | Life Science |
| Innovative Vibrational Spectroscopy Research for Forensic Application. Weberm A. et al.Analytical Chemistry, 2023 | Forensic |
| High-Throughput Antimicrobial Susceptibility Testing of Escherichia coli by Wide-Field Mid-Infrared Photothermal Imaging of Protein Synthesis. Guo, Z. et al.Analytical Chemistry, 2023 | Life Science |
| Prebiotic-Based Nanoamorphous Atorvastatin Attenuates Nonalcoholic Fatty Liver Disease by Retrieving Gut and Liver Health. Cui, J, et al.Small Structures, 2023 | Life Science |
| Optical photothermal infrared spectroscopy: A novel solution for rapid identification of antimicrobial resistance at the single-cell level via deuterium isotope labeling. Shams, S. et al.Front. Microbiol., 2023 | Life Science |
| Mapping ancient sedimentary organic matter molecular structure at nanoscales using optical photothermal infrared spectroscopy. Jubb, A. et al.Organic Geochemistry, 2023 | Paleontology |
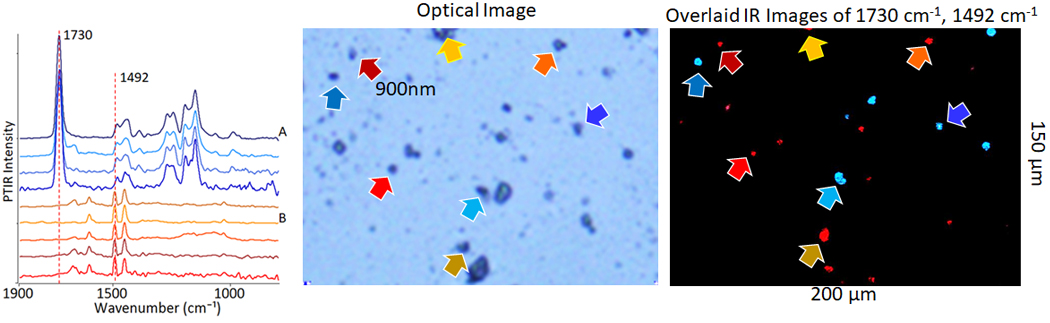
| A review on analytical performance of micro- and nanoplastics analysis methods. Thaiba, B.M. et al.Arabian Journal of Chemistry, 2023 | Microplastics |
| Video-rate Mid-infrared Photothermal Imaging by Single Pulse Photothermal Detection per Pixel. Xin, J. et al.bioRxiv, 2023 | Life Science |
| Microfluidics as a Ray of Hope for Microplastic Pollution. Ece, E. et al.biosensors, 2023 | Microplastics |
| Critical assessment of approach towards estimation of microplastics in environmental matrices. Raj, D. et al.Land Degradationa and Development, 2023 | Microplastics |
| Development of a Binary Digestion System for Extraction Microplastics in Fish and Detection Method by Optical Photothermal Infrared. Yan, F. et al.Frontiers in Marine Science, 2022 | Microplastics |
| Automated analysis of microplastics based on vibrational spectroscopy: are we measuring the same metrics?. Dong, M. et al.Analytical and Bioanalytical Chemistry, 2022 | Microplastics |
| Vitamin D and Calcium Supplementation Accelerate Vascular Calcification in a Model of Pseudoxanthoma Elasticum. Bouderlique, E. et al.International Journal of Molecular Sciences, 2022 | Pharmaceuticals |
| Polarization Sensitive Photothermal Mid-Infrared Spectroscopic Imaging of Human Bone Marrow Tissue. Mankar, R. et al.Applied Spectroscopy, 2022 | Biomedical and life science |
| Identification of spectral features differentiating fungal strains in infrared absorption spectroscopic images. Stancevic, D. et al.Lund Univ, Ugrad Thesis, 2022 | Bio and environmental |
| Optical photothermal infrared spectroscopy can differentiate equine osteoarthritic plasma extracellular vesicles from healthy controls. Clarke, E. et al.BioXvid, 2022 | BioXvid |
| Correlative imaging to resolve molecular structures in individual cells: substrate validation study for super-resolution infrared microspectroscopy. Paulus, A. et al.Nanomedicine: Nanotechnology, Biology, and Medicine, 2022 | Biomedical and life science |
| Leveraging high-resolution spatial features in mid-infrared spectroscopic imaging to classify tissue subtypes in ovarian cancer. Gajjela, C. et al.BioarXiv, 2022 | Biomedical and life science |
| APPLICATION OF OPTICAL PHOTOTHERMAL INFRARED (O-PTIR) SPECTROSCOPY TO ASSESS BONE COMPOSITION AT THE SUBMICRON SCALE. Reiner, E. et al.Temple Univ, Master thesis, 2022 | Biomedical and life science |
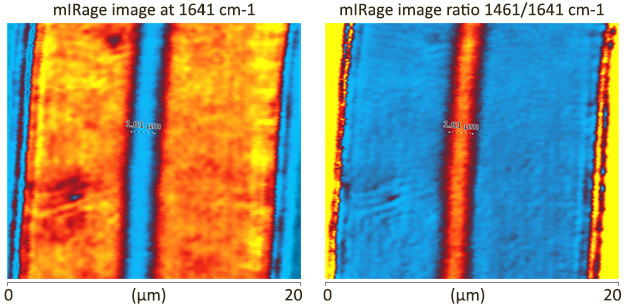
| Matrix/Mineral Ratio and Domain Size Variation with Bone Tissue Age: a Photothermal Infrared Study. Ahn, T. et al.Journal of Structural Biology, 2022 | Journal of Structural Biology |
| Simultaneous Raman and infrared spectroscopy: a novel combination for studying bacterial infections at the single cell level. Lime, C. et al.Chemical Science, 2022 | Biomedical and life science |
| Phase separation in surfactant-containing amorphous solid dispersions: Orthogonal analytical methods to probe the effects of surfactants on morphology and phase composition. Yang, R. et al.International Journal of Pharmaceutics, 2022 | Pharmaceuticals |
| Synovial joint cavitation initiates with microcavities in interzone and is coupled to skeletal flexion and elongation in developing mouse embryo limbs. Kim, M. et al.Biology Open, 2022 | Biomedical and life science |
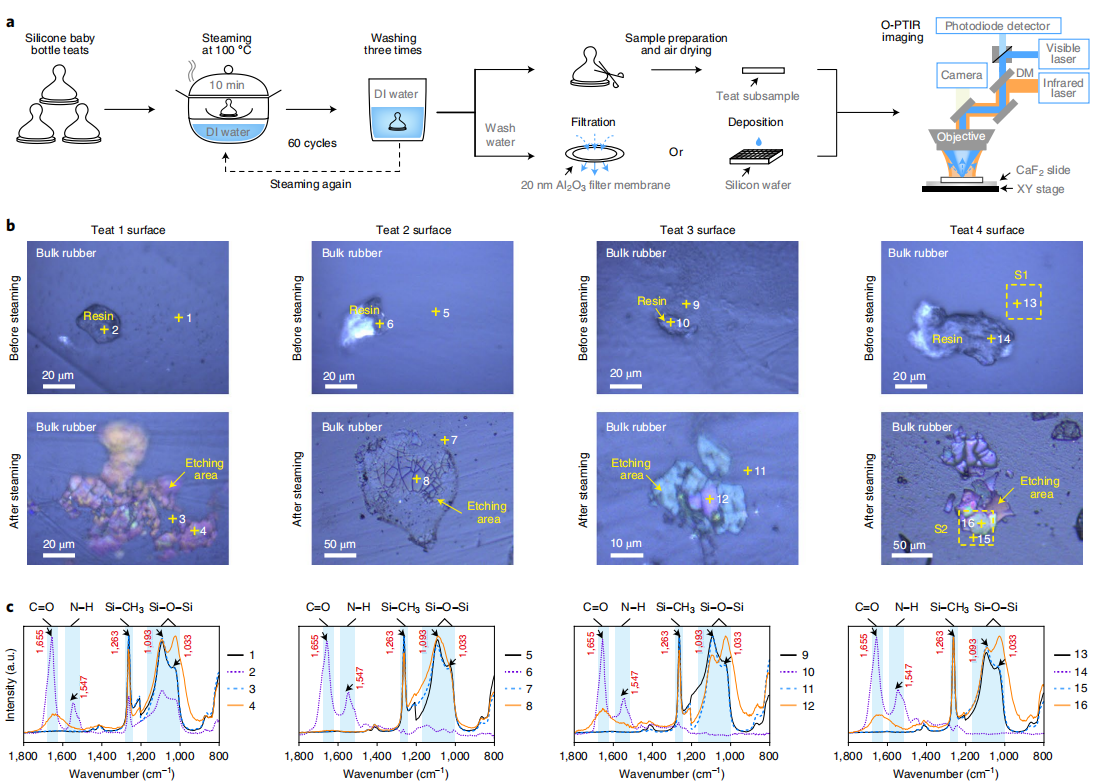
| Steam disinfection enhances bioaccessibility of metallic nanoparticles in nano-enabled silicone-rubber baby bottle teats, pacifiers, and teethers. Su, Y. et al.Journal of Environmental Science, 2022 | Microplastics |
| NOVEL SPECTROSCOPY TECHNIQUES USED TO INTERROGATE EQUINE OSTEOARTHRITIC EXTRACELLULAR VESICLES. Clarke, E. et al.Osteoarthritis and Cartilage, 2022 | Biomedical and life science |
| Using mid infrared to perform investigations beyond the diffraction limits of microcristalline pathologies: advantages and limitation of Optical PhotoThermal IR spectroscopy. Bazin, D. et al.Comptes Rendus. Chimie, 2022 | Biomedical and life science |
| Optical photothermal infrared spectroscopy can differentiate equine osteoarthritic plasma extracellular vesicles from healthy controls. Clarke, E. et al.Analytical Methods, 2022 | Biomedical and life science |
| Probing Individual Particles Generated at the Freshwater–Seawater Interface through Combined Raman, Photothermal Infrared, and X-ray Spectroscopic Characterization. Mirrielees, J. et al.ACS Meas. Sci. Au, 2022 | Environmental and Microplastics |
| Parts-per-Million Detection of Trace Crystal Forms Using AF-PTIR Microscopy. Razumtcev, A. et al.Analytical Chemistry, 2022 | Pharmaceuticals |
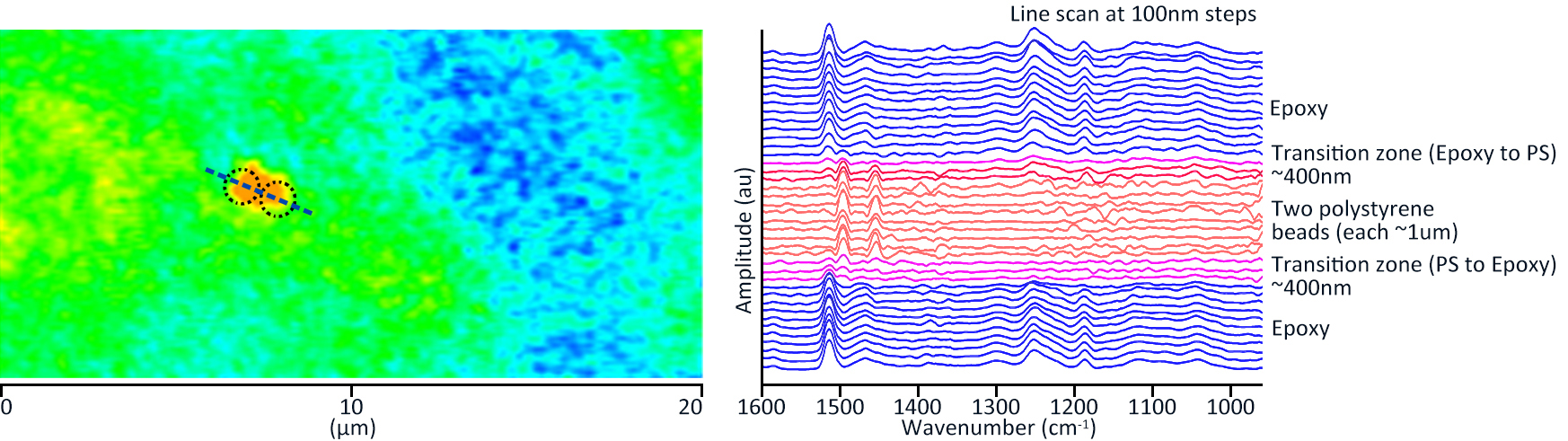
| Ultrafast Widefield Mid-Infrared Photothermal Heterodyne Imaging. Paiva, E. et al.Analytical Chemistry, 2022 | Photonics, bio |
| Chapter 8 - Raman-integrated optical photothermal infrared microscopy: technology and applications. Li, X. et al.Molecular and Laser Spectroscopy, 2022 | Photonics, bio |
| Chapter 9 - Optical photothermal infrared spectroscopic applications in microplastics—comparison with Fourier transform infrared and Raman spectroscopy. Krafft, C. et al.Molecular and Laser Spectroscopy, 2022 | Microplastics |
| Contribution of Infrared Spectroscopy to the Understanding of Amyloid Protein Aggregation in Complex Systems. Ami, D. et al.Front. Mol. Biosci., 2022 | Bio and life science review |
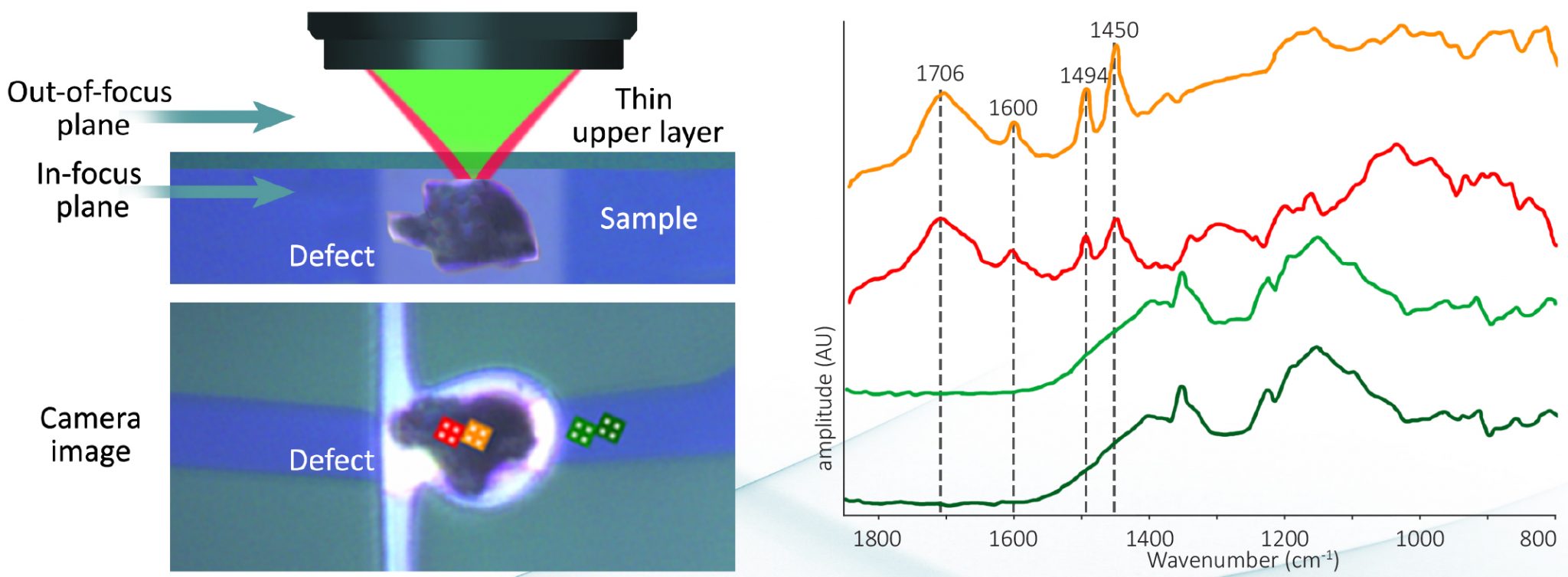
| Novel Submicron Spatial Resolution Infrared Microspectroscopy for Failure Analysis of Semiconductor Components. Zulkifli, S. et al.IPFA 2022 Proceedings, 2022 | FA/contamination |
| Overcoming challenging Failure Analysis sample types on a single IR/Raman platform. Anderson, J. et al.ISTFA 2022 Proceedings, 2022 | FA/contamination |
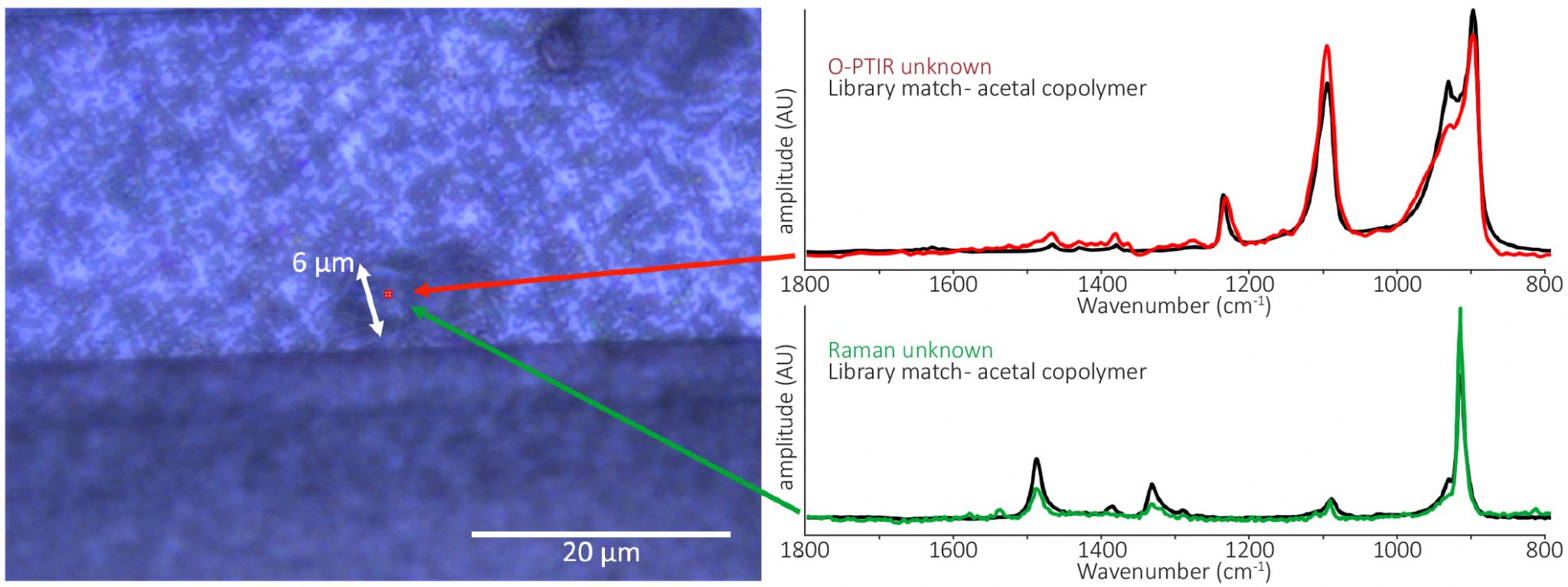
| Optical photothermal infrared spectroscopy with simultaneously acquired Raman spectroscopy for two-dimensional microplastic identification. Boeke, J. et al.Scientific Report, 2022 | Microplastics |
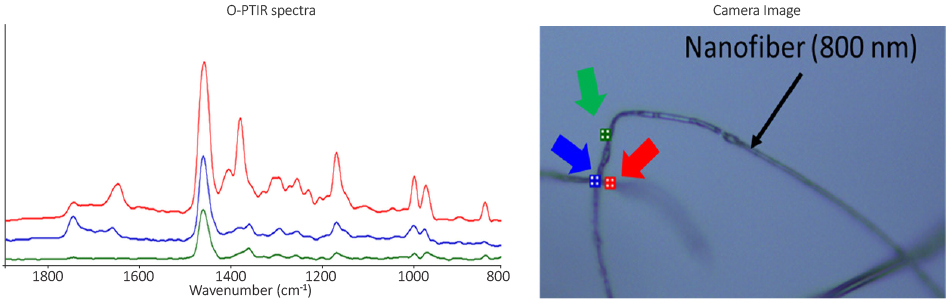
| Super-resolution infrared microspectroscopy reveals heterogeneous distribution of photosensitive lipids in human hair medulla. Sandt, C. et al.Talanta, 2022 | Life science, hair |